Featured
- Get link
- X
- Other Apps
Hydrogen Bonds Form Between Neighboring Water Molecules Because Of
Hydrogen Bonds Form Between Neighboring Water Molecules Because Of. These bonds are transient and easily and quickly shift among molecules. Hydrogen bonds form between neighboring water molecules because of:
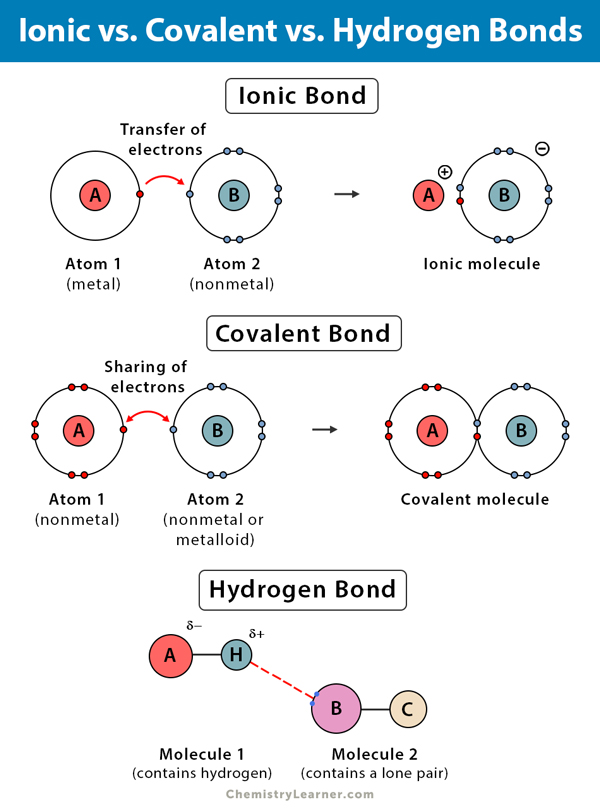
Covalent bonds form between hydrogen and oxygen atoms in a water molecule as a result of the: Each water molecule forms multiple hydrogen bonds with neighboring water molecules. Hydrogen bonds make water sticky in the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules.
Water Molecules Stick To Other Materials Due To Its Polar Nature.
Hydrogen bonds form between neighboring molecules. Each water molecule is joined to other. The key to understanding water’s chemical behavior is its molecular structure.
Covalent Bonds Form Between Hydrogen And Oxygen Atoms In A Water Molecule As A Result Of The:
A hydrogen bond in water occurs between the hydrogen atom of one water molecule and the lone pair of electrons on an oxygen atom of a neighboring water molecule. Because of the polarity of the molecules, water molecules are attracted to each other. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons.
What Are The 4 Types Of Bonds?
How many bonds are in co2? Due to the electronegativity difference between the atom pairs mentioned, electrons are unevenly shared across the covalent bond. The attraction between individual water molecules creates a bond known as a hydrogen bond.
Sharing Of Electrons Between Atoms.
Generally, a proton is shared by two lone electron pairs. Cohesion is a key property of water. What are the bonds between water molecules called?
The Image Above Depicts Water Molecules.
Because of its cohesiveness, water remains a liquid at normal temperatures rather than vaporizing into a gas. The attraction between individual water molecules creates a bond known as a hydrogen bond. These strong intermolecular forces are formed between water molecules and are responsible for the high boiling point and wide range of temperatures in liquid water.
Comments
Post a Comment